A novel DNA-based drug delivery system for HER2-Positive Breast Cancer: safe firstly and targeting dually
Researchers from West China School of Stomatology (WCSS), Sichuan University have developed a dual-targeting nanoparticle by combining pH-sensitive camouflage and HApDC with superior safety, initiating an important step toward the development and application of DNA-based medicine and biomimetic cell membrane materials in cancer treatment and other potential biological applications.
Their research <https://doi.org/10.1002/adma.202108300> was published in the journal Advanced Materials.
In a previous study, the group modified anti-HER2 protein aptamer (HApt) with tetrahedral framework nucleic acid (tFNA) to form HApt-tFNA, which has excellent affinity for HER2-positive breast cancer cells. In this study, the group modified the chemotherapeutic drug Maytansine (DM1) on multiple single chains of HApt-tFNA as a novel aptamer-drug conjugate (ApDC), and HTD will deliver more DM1 (DM1/ HApt-tFNA=3:1). HTD has higher affinity and cytotoxicity for HER2-positive breast cancer cells than HER2-negative breast cancer cells and normal breast cells. The affinity and cytotoxicity of HTD to HER2 negative breast cancer cells and normal breast cells are poor, and it can reduce the toxicity and side effects of DM1 to normal cells. The hybrid erythrosome-based nanoparticles show better inhibition of HER2-positive cancer than free HTD in vivo. With the strengths of precise delivery, increased drug loading, sensitive tumor probing, and prolonged circulation time, the PEOz-erythrosome@HTD represents a promising nanomedicine to treat HER2-positive tumors.
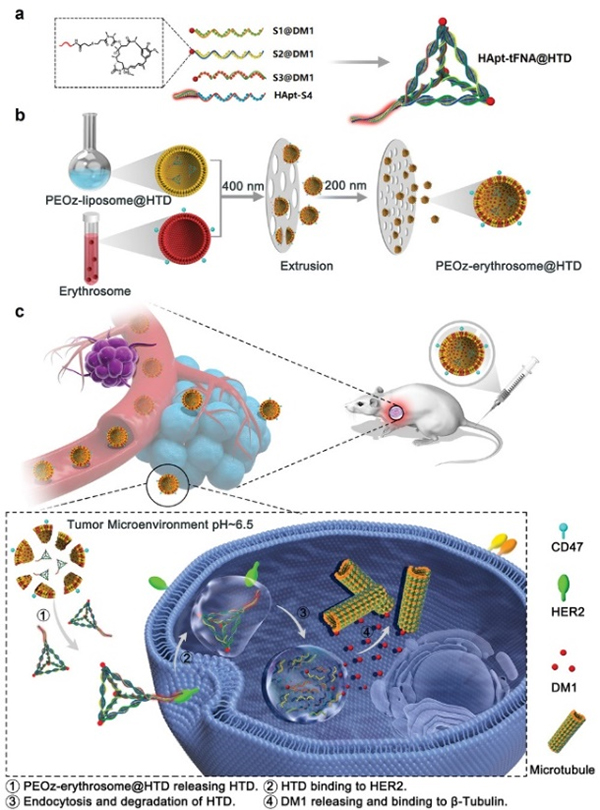
Schematic illustration of the synthesis of HApt-tFNA@DM1 (HTD), PEOz-erythrosome@HTD, and their proposed antitumor mechanism.
Their findings, published in ADVANCED MATERIALS on 22 January 2022 created a bionic, pH-sensitive erythrocyte membrane-artificial lipid hybrid nanocarrier (PEOz-erythrosome@HTD), capable of targeted delivery and release of HTD in response to the tumor microenvironment, increasing the circulation time of HTD and drug concentration in target tissues.
The study was conducted by Professor Yunfeng Lin and his team. Dr. Wenjuan Ma and Dr. Yuting Yang are the co-first authors. Professor Lin is the corresponding author.
For more details, please click the link below.
https://onlinelibrary.wiley.com/doi/10.1002/adma.202109609




